vespr chart|vsepr electron geometry chart : Tuguegarao VSEPR theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. A central atom is defined in this theory as an atom which is bonded to two or more other atoms, while a terminal atom is bonded to only one other atom. For example in the molecule methyl isocyanate (H3C-N=C=O), the two carbons an. DATALOG LIMITED - Free company information from Companies House including registered office address, filing history, accounts, annual return, officers, charges, business activity
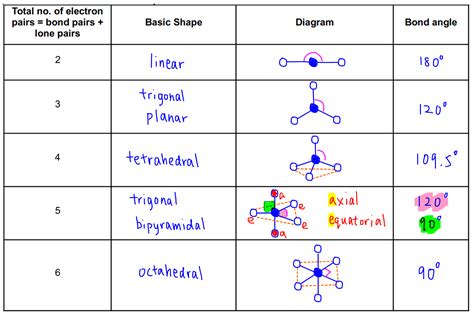
vespr chart,Use our handy VSEPR chart to find the 3-D geometric VSEPR shapes of molecules and ions and learn about VSEPR theory and shapes.The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and .
Learn how to use VSEPR theory to organize molecules based on their geometric structures and bond angles. See examples of VSEPR geometries with and with.VSEPR theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. A central atom is defined in this theory as an atom which is bonded to two or more other atoms, while a terminal atom is bonded to only one other atom. For example in the molecule methyl isocyanate (H3C-N=C=O), the two carbons an.Learn how to use the VSEPR theory to predict the molecular shape and geometry of polyatomic molecules. See the AXE notation, the basic molecular structures, and the examples of VSEPR chart.
Learn how to use VSEPR (valence shell electron pair repulsion) theory to predict the 3-D shape and bond angle of molecules and ions. See examples, definitions, and limitations of VSEPR model.

The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal .Summary VSEPR and Hybridization Table. Electron Domains. Electron-Domain Geometry. Predicted Bond Angle(s) Hybridization of Central Atom.
VSEPR stands for valence shell electron pair repulsion. Valence shell electrons are on the outer part of an atom. These electrons can participate in bonding (bonding pairs) or act .
vsepr electron geometry chart Molecular Models (VSEPR Theory) - University of Illinois Urbana-Champaign . NextThus according to the VSEPR model, the C–N=C fragment should be bent with an angle less than 120°. The carbon in the –N=C=O fragment is doubly bonded to both nitrogen and oxygen, which in the VSEPR model gives carbon a total of two electron pairs. The N=C=O angle should therefore be 180°, or linear.
We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons .
The valence shell electron pair repulsion model is often abbreviated as VSEPR (pronounced "vesper") and is a model to predict the geometry of molecules. Specifically, VSEPR models look at the .

The valence shell electron pair repulsion (VSEPR) theory is a model used to predict 3-D molecular geometry based on the number of valence shell electron bond pairs among the atoms in a molecule or ion. This model assumes that electron pairs will arrange themselves to minimize repulsion effects from one another.vespr chart vsepr electron geometry chartThe valence shell electron pair repulsion (VSEPR) theory is a model used to predict 3-D molecular geometry based on the number of valence shell electron bond pairs among the atoms in a molecule or ion. This model assumes that electron pairs will arrange themselves to minimize repulsion effects from one another.
2.2.1. VSEPR. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.
VSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .
vespr chartVSEPR Theory. Valence Shell Electron Pair Repulsion (VSEPR) is a theory that states that the 3d orientation, also known as the molecular geometry, of a molecule is not dependent on its chemical formula but on the repulsion of valence electrons.In other words, two molecules with the general formulas `AB_3` may look completely different in real life: .The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt .
vespr chart|vsepr electron geometry chart
PH0 · vsepr shape chart
PH1 · vsepr model simulator
PH2 · vsepr model chart
PH3 · vsepr electron geometry chart
PH4 · vsepr diagram generator
PH5 · vsepr diagram
PH6 · vsepr chart with hybridization
PH7 · vsepr chart with angles
PH8 · Iba pa